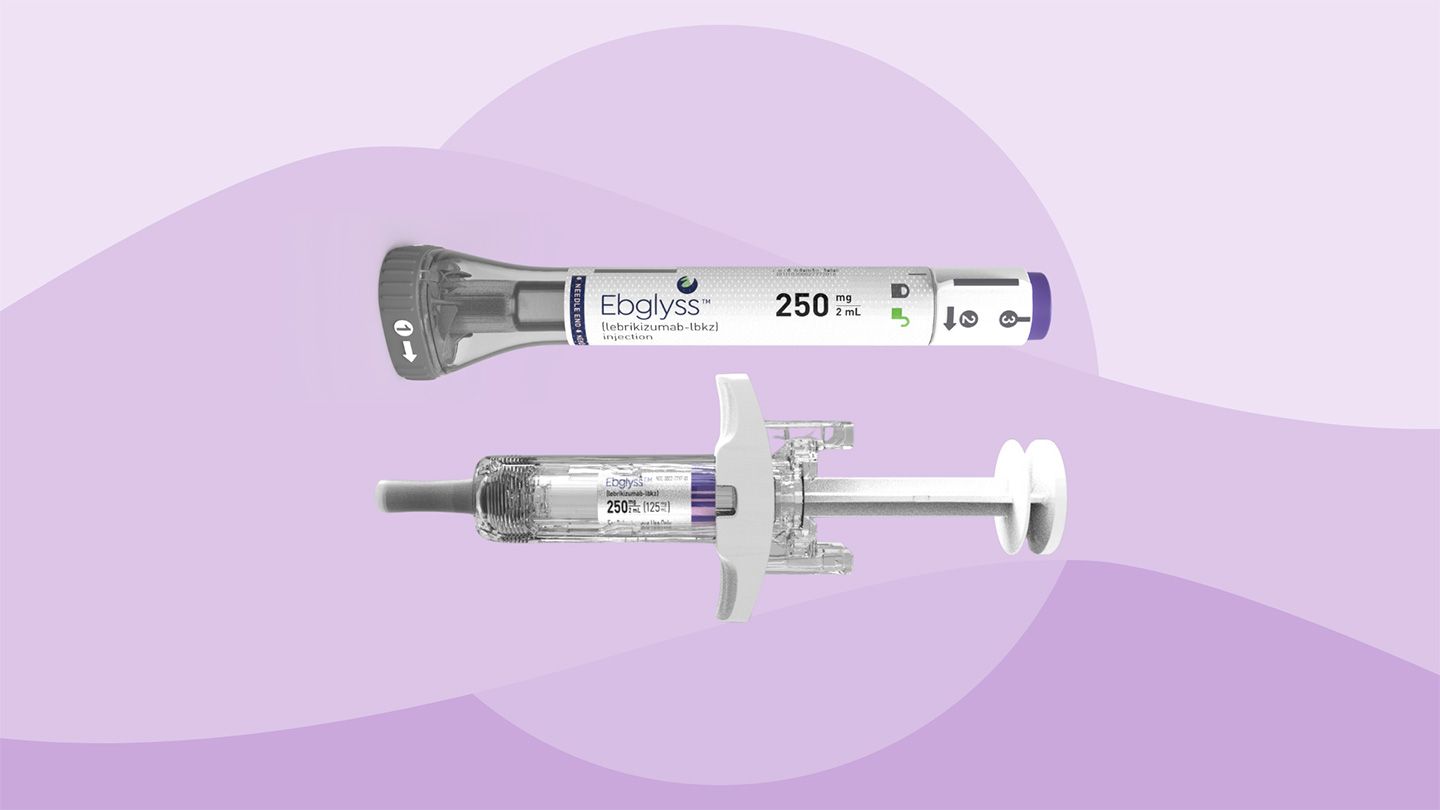
People with moderate-to-severe atopic dermatitis, the most common form of eczema, don’t always get adequate relief from topical treatments like steroid creams and ointments. Now they have a new option: The U.S. Food and Drug Administration (FDA) just approved Ebglyss (lebrikizumab-lbkz), a biologic treatment for adults and children ages 12 years and older.
This once-a-month injectable drug belongs to a class of medicines called targeted interleukin-13 (IL-13) inhibitors, and offers a new systemic option to combat the underlying inflammation in people living with atopic dermatitis, clearing skin and alleviating itch.
The approval of Ebglyss has been highly anticipated because the data on the phase 3 studies is so strong, says Amy McMichael, MD, a dermatologist who specializes in treating people of color with dermatologic conditions at Atrium Health Wake Forest Baptist in Winston Salem, North Carolina. (Dr. McMichael was not involved in any of this research.) One study that focused exclusively on people of color determined that the medication provided consistent results no matter an individual’s skin tone.
“People can have a reduction in signs of their disease, relief from itch, and are able to reimagine what they can do with their lives now that they can function better,” says Mark Genovese, MD, senior vice president of immunology development at Eli Lilly, the drug’s manufacturer.
Nearly 40 Percent of People Achieved Clear Skin in 16 Weeks
The approval of Ebglyss is based on three pivotal phase 3 trials, ADvocate 1, ADvocate 2, and ADhere, which included a total of over 1,000 participants ages 12 and older with moderate-to-severe eczema who were unable to control their symptoms with topical prescription medications.
Key findings from the ADvocate 1 and 2 trial included:
Skin Clearance In an average of Advocate 1 and 2, 38 percent of participants achieved clear or almost-clear skin at 16 weeks with Ebglyss (versus 12 percent with placebo).
Ten percent of people taking Ebglyss for four weeks achieved clear or almost-clear skin.
Long-Term Efficacy Of the people who experienced cleared skin, 77 percent maintained it at one year with once-monthly dosing.
Additionally, 48 percent of those who were switched to placebo at week 16 maintained their improvement at one year.
Itch Relief Significant itch relief at 16 weeks was experienced by 43 percent of participants, with some people reporting relief as soon as two weeks into treatment.
Side Effects The most common side effects of Ebglyss included eye and eyelid inflammation, such as redness, swelling, and itching; injection site reactions; and shingles (herpes zoster).
Ebglyss Proves Effective in People of Color
In a first-of-its-kind study, Ebglyss showed improvement in skin clearance and itch relief in a small trial of 50 people with darker skin tones, which included Black, Asian, Hispanic/Latinx, and American Indian or Alaska Native people. The results have yet to be published in a peer-reviewed journal.
Results at 16 weeks were consistent and similar with earlier trials, with most people experiencing significant improvement, and close to 40 percent achieving clear or almost-clear skin.
People of color are disproportionately affected by atopic dermatitis, often experiencing more severe symptoms, a delay in diagnosis, and a lengthier time frame to find appropriate treatment, according to lead author Andrew Alexis, MD, MPH, a professor of clinical dermatology at Weill Cornell Medicine and a dermatologist at NewYork-Presbyterian/Weill Cornell Medical Center, both in New York City.
A recent national health survey found that about 19 percent of Black children have atopic dermatitis, compared with about 16 percent of white children and 8 percent of Asian children. Another study found that Black children are 1.7 times more likely to develop atopic dermatitis than white children.
“They also have been historically underrepresented in clinical trials, which means we have lacked data pertaining to the treatment of patients with skin of color,” said Dr. Alexis.
Because most dermatologic trials often don’t include people of color, providers have to assume medications work in all races and ethnicities — even without the data, says McMichael.
“This trial in patients of color shows that this drug is unequivocally effective in patients with skin of color,” she says.
How Much Will Ebglyss Cost?
The list price in the United States for Ebglyss is $3,500 per pen, according to a Lilly spokesperson.
Without insurance, that means a person on a typical dosing schedule would spend $38,500 by week 16.
Ebglyss will be available in the United States in the coming weeks, according to the company press release.